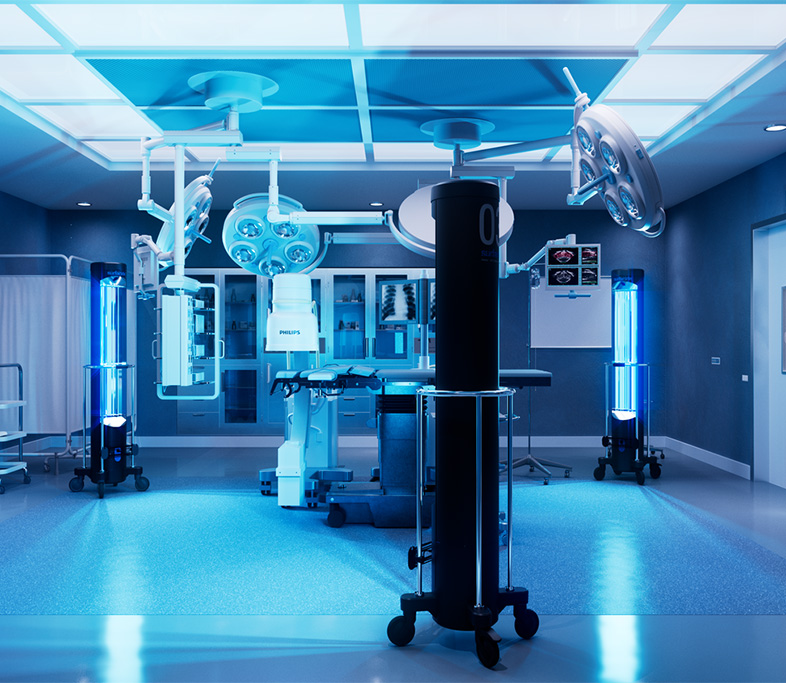
University Of Iowa Study is First Clinical Study evaluating UV-C efficacy against SARS-CoV-2 in Hospital Operating Rooms Proving Surfacide Technology Makes Environments Safer for Healthcare Workers and Patients
A recent peer-reviewed study in the Journal of Clinical Anesthesia* found the Surfacide Helios® System UV-C technology is nearly 95% effective in preventing SARS CoV-2 in patient environments. This is the first clinical study that evaluates the use of UV-C in operating rooms, compared to previous studies conducted in a controlled lab environment.
The study was led by Dr. Randy Loftus, MD, an Associate Professor of Anesthesiology and Critical Care Medicine at University of Iowa Hospital and Clinics, and Dr. Franklin Dexter, MD, PhD, University of Iowa Professor in the Department of Anesthesia. Dr. Loftus has received national and international recognition for his expertise regarding the epidemiology of intraoperative pathogen transmission. In the last 10 years he has made numerous contributions to science as is reflected in a solid body of published evidence, including a focus on infection prevention protocols in the perioperative space. Dr. Dexter and his colleagues have developed much of the science in anesthesia group and operating room management.
For this study, Surfacide’s Helios System was deployed—the world’s first and only patented trio of UV light-emitting ‘robots’ that simultaneously provide floor to ceiling UV-C energy. A total of 473 intraoperative reservoir samples were tested among 13 anesthesia work areas and 12 environmental locations. The following results were confirmed:
- Environmental locations tested monitored proximal sites exposed to droplet spread and more distal sites potentially impacted by aerosolized particles.
- Use of Surfacide UV-C disinfection showed nearly a 2-fold reduction in SARS-CoV-2 detection as compared to traditional surface disinfection cleaning procedures without UV-C.
- Surface disinfection cleaning alone is associated with greater environmental detection of SARS-CoV-2.
- The Surfacide technology was associated with decreased residual contamination of distal sites impacted by aerosolization.
“This study further validates Surfacide’s effectiveness as a critical infection control measure in combating deadly pathogens in real world environments,” said Surfacide Founder and CEO Gunner Lyslo. “Surfacide’s triple-emitter, floor to ceiling UV technology, along with its effectiveness in inactivating aerosolized pathogens AND pathogens colonizing surfaces, makes this study a game-changer in infection prevention and confirms that not all UV-C is created equal.”
The data presented in this paper emphasizes the importance of Surfacide UV-C as an infection control measure against pathogens found on surfaces and in the air, especially within the operating room space. Surfacide’s evidence-based approach is critical in providing a safer environment for healthcare staff and patients alike. It’s proven ability, as documented in this study, sets this technology apart from all other UV devices.
*R.W. Loftus, F. Dexter, L.C. Evans, et al., An assessment of the impact of recommended anesthesia work area cleaning procedures on intraoperative SARS-CoV-2 contamination, a case-series analysis, Journal of Clinical Anesthesia (2021)
About SurfacideSurfacide LLC and Surfacide Manufacturing Inc. are located in Waukesha, WI and produce multiple emitter UV-C systems, all made in the USA. Surfacide is the only patented triple emitter UV-C system, providing the most efficient and flexible solution in the fight against viruses and bacteria that cause hospital-acquired infections. Hundreds of hospitals and venues worldwide are using Surfacide technology to provide an extra layer of protection for patients, healthcare workers and consumers alike. Surfacide’s evidence-based UV-C solution reaches more surfaces, reduces shadowed areas, and requires less labor for a quicker turnaround time and better efficacy. Learn more about Surfacide by visiting https://surfacide.com or calling (844)-390-3538.